Lesson 3: Chemical Reactions and Equations
2. Reaction Types
1.Single Replacement
In these reactions, one of the elements in the compound is replaced by another element.
Cu(s) +AgNO3(aq)--> Ag(s) +Cu(NO3)2(aq)
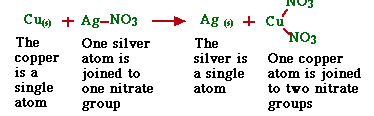
2. Double Replacement
In these reactions, both the metal and nonmetal will be replaced between two compounds.
Mg(OH)2(s) + HCl(aq)--> MgCl2(aq) +HOH(l)

3. Synthesis Reaction
A synthesis reaction, in the simplest sense, involves elements as reagents and the formation of a compound (a substance composed of more than one element) as the product, often as the only product.
Fe + S ---> FeS
Iron + Sulfur = Ion sulfide
4. Decomposition
Decomposition reactions are chemical reactions in which chemical species break up into simpler parts.
E.g. 2NH3 → N2 + 3H2
Compounds don't need to break down into elements in a decomposition reaction.
E.g.
(NH4)2CO3(s) →2NH3(g)+CO2(g) +H2O(g)
5. Hydrocarbon Combustion
When hydrocarbons such as gasoline, methane, and sucrose are burned (combusted), they always produce energy, carbon dioxide gas, and water vapor. The act of burning is actually just a rapid reaction with oxygen.
E.g. methane gas is burned (combusted).
CH4(g) + 2O2(g) →CO2(g) + 2H2O(g) +Energy
A Final Note...
There are other categories of chemical reactions. The two broadest categories are the acid-base reactions and oxidation-reduction reactions.
You will learn more about the former in the following units, and the latter in Chemistry 30.