Lesson 3: Chemical Reactions and Equations
3. Balancing Chemical Reactions
A chemical equation is a way to describe what goes on in a chemical reaction, the actual change in a material. Chemical equations are written with the symbols of materials to include elements, ionic or covalent compounds, aqueous solutions, ions, or particles. The following diagram shows the components of a chemical reaction.
![]()
Suppose you are camping and have been sitting around a fire all night. You have burned an awful lot of wood. You get up the next morning only to find that there are ashes left. Where did all the wood go? This seems to contradict the law of conservation of mass. In reality, most of the mass has been given off as water vapor and carbon dioxide. If you could collect the water vapor, carbon dioxide, heat, and all the ash, you would find that it would equal the same mass as the wood you burned. This law ties in well with the atomic theory of matter. According to the theory, atoms are never created or destroyed. In chemical reactions, the atoms and molecules are simply rearranged. Molecules may be broken apart and new ones may be formed, but the atoms in the products are the same ones that were in the reactants.
Often you will see the descriptions of the materials in the reaction in parentheses after the material. Gas is shown by (g). Solid material is shown by (s). Liquid is shown by (l). A material dissolved in water (an aqueous solution) is shown by (aq). This when balancing chemical reactions comes to play. Now, let's see how we can balance reactions with a few examples.
Example 1. Mg(s) + O2(g) → MgO(s)
The formula, MgO, indicates that for every magnesium atom there is an oxygen atom. We start out with one magnesium atom and two oxygen atoms, ending up with one magnesium atom and one oxygen atom. What happens to the other oxygen? It must be accounted for!
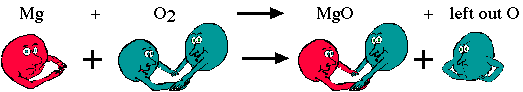
When the oxygen molecules break apart to react with the magnesium atom, the lone oxygen is free to bond. We assume that the only elements present are those listed. This means that oxygen can bond with another magnesium. This will make all of the atoms more stable.
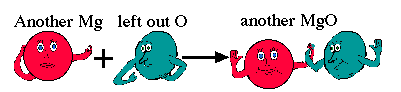
In other words, we have now used up two magnesium atoms and created two units of magnesium oxide. We show this by placing numbers in front of the molecule.
2Mg(s) + O2(g) → 2MgO(s)
This is a balanced equation. It can be read as follows: Two atoms of magnesium react with one molecule of oxygen to form two molecules of magnesium oxide.
The goal is to ensure that the same number of the same type of atoms appear on each side of the equation. To balance an equation you may start with any atom but you may find it helpful to start with the one present in higher numbers.
Example 2. S8(s) + Li → Li2S(s)
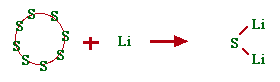 Start with the sulfur atoms. 8 on the reactant side means we can make 8 units of Li2S(s). We must place an 8 in front of the Li2S. The number of lithium atoms required to make 8 units of Li2S(s) is 16. We must then have 16 on the reactant side. The equation becomes:
Start with the sulfur atoms. 8 on the reactant side means we can make 8 units of Li2S(s). We must place an 8 in front of the Li2S. The number of lithium atoms required to make 8 units of Li2S(s) is 16. We must then have 16 on the reactant side. The equation becomes:
S8(s) + 16Li → 8Li2S(s)
These two examples lead us to the following three steps when balancing chemical equations:
Steps For Balancing Chemical Reactions:
1. Write a word equation for the reaction
2. Write the correct formula for the reactants and products.
3. Determine coefficients that make the equation balanced.
Example 3. H2O → H2(g) + O2(g)
You can, of course, balance your reactions, using the method you want, but I would like to suggest a method for you to use is the Balance (B) & Count (C) Method.
Let's balance the above reaction using this method.
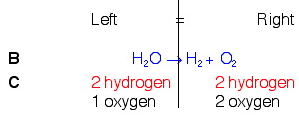
So, looking back at our rules, knowing that we can only add coefficients at the beginning of compounds, and knowing that we need a total of 2 oxygen on the left, we will put a 2 in front of water on the left. Once we do this, we will count again and see if we need to do anything else.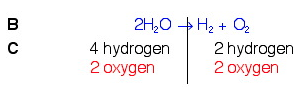
In balancing the oxygens, we changed the number of hydrogens on the left. This is OK. But we still need to balance them. To do this, we will put a 2 in front of the hydrogen count on the right. This should fix the problem.
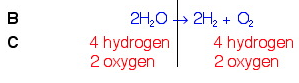
We now see that the equation is balanced.
To sum up, the Law of Conservation of Mass states that in a chemical reaction there is no loss of mass. Each type of element will have the same amount before the reaction and after the reaction, or as reactant and product. But you can't change the materials that participate in the reaction, so you must write an integer coefficient in front of each material in the reaction to ensure every type of atom has the same number on each side of the reaction.