Lesson 4: Chemical Bonding and Diversity of Matter
2. Bonding Theory and Lewis Symbols
Electron pairing is an important concept in understanding how chemical bonds occur. Electrons occupy orbitals. Orbitals are defined as areas of space where an electron of a given energy is most likely to be found.
An orbital can hold a maximum of 2 electrons. Since electrons repel each other, electrons will occupy empty valence orbitals before pairing up.
Paired electrons in an orbital are called lone pairs. Unpaired electrons in an orbital are called bonding electrons. The number of bonding electron is called the bonding capacity of the atom. Atoms with a stable bonding condition will have 2 elections in each of their 4 valence orbitals, as the situation called a stable octet or octet rule. Only the valence electrons of atoms are involved in bonding. Valence electrons are the electrons that are found in the highest energy level of atoms.
Explaining Molecular Formulas Using Lewis Symbols:
Lewis symbols ( electron-dot diagrams) are a method of keeping track of the valence electrons of atoms. In these diagrams, the element's symbol represents the nucleus and inner electrons of the atom. Each side of the symbol represents a valence orbital.
Elements in the same group of the periodic table will have a similar electron symbol because they have the same number of valence electrons. Watch the following video explaining these concepts diagrammatically.
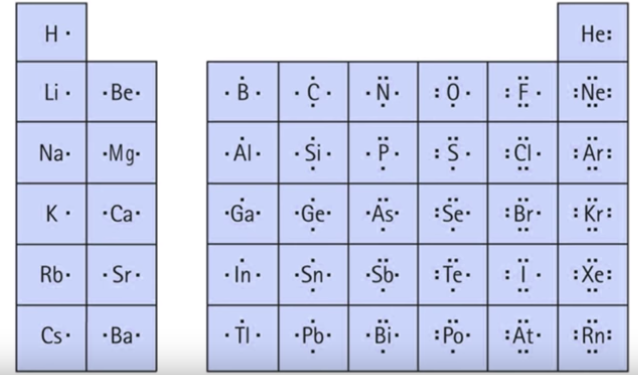
For example, the C atom has 4 valence electrons, 4 bonding, and 0 lone pair. Sulfur has 2 lone pairs and 2 bonding e-.
Molecular Elements:
There are seven elements are diatomic: Br2, I2, Cl2, H2, O2, F2. For example, Fluorine has seven valence electrons, requiring one more electron to complete its octet be become stability, meaning fill its orbitals.
![]() In the following video, I will show you how the three of these diatomic molecules are forming bonds to satisfy octet rule and duet rules in H2.
In the following video, I will show you how the three of these diatomic molecules are forming bonds to satisfy octet rule and duet rules in H2.