Lesson 2. Concentration of Ionic Solutions
.
2. Ionization
Ionization
Earlier, we talked about ions produced via dissociation which refers to the separation of ions that are present in ionic compounds. Now, we will study the concept of ionization which refers to the reaction in which the polar covalent compounds are converted into ions in water.
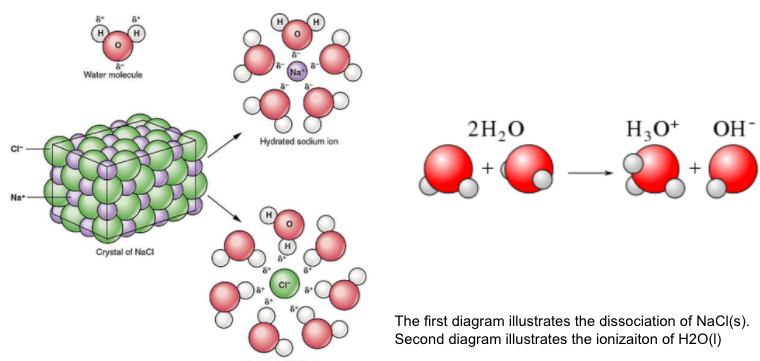
When molecular solute ionizes in solution the molecular solute reacts with the solvent (water) to form ions not present in the original molecule. The best example of ionization can be seen in strong and weak acids and bases.
HCl(aq) + H2O(l) --> H3O+(aq) + Cl-(aq)
NH3(g) + H2O(l) --> NH4+(aq) + OH-(aq)
In both examples, none of the ions formed existed in the original solute of HCl and NH3. We use dissociation and ionization equations to calculate the concentration of individual ions in solution. In a balanced equation, the coefficients of the ions produced in solution will give us ratios of concentration relative to the concentration of the solute.
Example 1
What is the concentration Na+(aq) and PO43- in a solution of 0.600 mol/L Na3PO4(aq)?
Solution
Step 1: Write a balanced dissociation equation.
Na3PO4(s) --> 3Na+(aq) + PO43-(aq)
From the equation, there will be 3 moles of Na+ for every 1 mole of Na3PO4(s).
Step 2: Calculate the concentration of Na+(aq) and PO43-(aq). Use square brackets to represent the concentration
[Na+(aq) = 3x 0.600 mol/L = 1.800 mol/L
[PO43-(aq)] = 1x 0.600 mol/L = 0.600 mol/L
Example 2
Determine the amount concentration of barium and hydroxide ions in a solution made by dissolving 5.48 g of barium hydroxide to make a volume of 250 mL.
Solution
Step 1: Write a balanced ionization reaction, and list givens
Ba(OH)2(aq) --> Ba+(aq) + 2OH-(aq)
5.48g
171.35 g/mol (molar mass)
250 ml= 0.250 L
Step 2: calculate moles: molar mass of barium hydroxide: 171.35 g/mol
n = 4.48g x 1mol/ 171.35 g = 0.032 mol Ba(OH)2
C= n/ V = 0.032/ 0.250 = 0.128 mol/L
Step 3: Use mole ratio which is (coefficients) to determine the concentration
Ba(OH)2(aq) --> Ba+(aq) + 2OH-(aq)
1 mol/L 1 mol/L 2 mol/L
0.128 mol/L 0.128 mol/l 0.256 mol/L