Module 2 Intro
1. Module 2 Intro
1.24. Page 6
Module 2—Chemical Compounds
 Reflect and Connect
Reflect and Connect
At the beginning of this lesson, you considered the function of antifreeze in an automobile engine. Engine coolants often contain alcohol compounds like ethane-1,2-diol, pictured here.
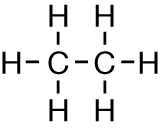
An antifreeze, or engine coolant, is responsible for transferring heat from the engine by circulating it through the radiator. Antifreeze is a solution containing mainly water and the coolant molecule. The boiling point of antifreeze is much higher than pure water. Knowing what you do about chemical structures and intermolecular bonds, can you suggest a reason?
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Place your answer to the question posed above in the Reflect and Connect section into question 3 of the Lesson 4 Assignment. Save your assignment to your course folder.
 Going Beyond
Going Beyond
You may be interested in cloud seeding—a process that takes place in Alberta, particularly in the corridor between Red Deer and Calgary. For more information read “Web Quest—Cloud Seeding” on page 112 of your textbook.
You may also be interested in the case study “Current Research on Intermolecular Forces” on pages 114 and 115 of your textbook. This case study examines the work of Canadian scientist Dr. Robert J. Le Roy.
 Module 2: Lesson 4 Assignment
Module 2: Lesson 4 Assignment
Retrieve the copy of the Module 2: Lesson 4 Assignment that you saved to your course folder.
Complete questions 4, 5, and 6 of the Lesson 4 Assignment. Save your work to your course folder. Send a copy of the completed Lesson 4 Assignment to your teacher.