Module 4 Intro
1. Module 4 Intro
1.6. Page 4
Module 4—Properties of Solutions
Aesthetic Qualities of Drinking Water
When you fill a glass with tap water, there are expectations for what this water should be like. You would not expect dirty or cloudy water; and if there were a bad smell, you might feel the water is unsuitable for drinking. There are a number of physical characteristics that must be inspected to make sure the water you drink meets guidelines for aesthetic quality.
Turbidity
turbidity: the measure of the clarity of a clear liquid
Turbidity is a measure of the water cloudiness that results from the suspension of small particles. In nature, water is continuously picking up sediment and organic matter. While the particles themselves may not pose a significant health risk, dangerous micro-organisms can attach themselves to these particles. During water treatment the sediment can actually protect the micro-organisms from disinfectant processes. If water is sterilized using ultraviolet (or UV) light, a micro-organism shielded by the sediment will not be destroyed.
Not all cloudy water is associated with turbidity. For example, tap water is sometimes cloudy when it first comes out of the tap, but clears up after it stands for a few minutes. This is due to air that is trapped in the water from turbulence in the pipes. This type of cloudiness has no health concerns associated with it.
Colour
Most people expect their drinking water to be clear, but sometimes there are substances present that give the water a tint. In Canada, 90% of the drinking water comes from surface water, while the remaining 10% comes from groundwater. If surface water has a colour, it likely results from organic substances of natural origin. If groundwater has a colour, it likely results from dissolved minerals. One of the main problems with coloured water is that it can stain laundry and plumbing fixtures.
Water Tint |
Common Cause |
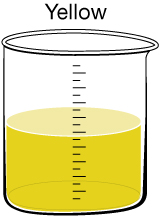 |
Humic Acid Discolouration.This discolouration can be caused by humic acid, which is present naturally in soil and vegetation. At the current time, it is assumed this substance has no significant effect on human health. Research is being done to see whether the chlorination process reacts with humic acid to create toxic by-products or whether the presence of humic acid correlates to the presence of heavy metals in the drinking water. |
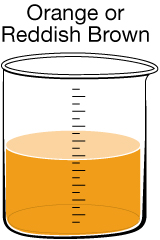 |
Iron Discolouration. Iron can naturally enter drinking water from soil and rocks or from being leached out of pipes. In low doses iron is a nutrient required by the body, so there is no significant health risk.
Reddish-brown water can also occur if rust-coloured sediments are disturbed during water main repairs. This discolouration can often be remedied by letting the tap run for several minutes. |
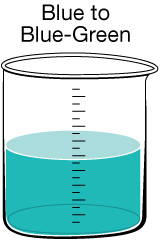 |
Copper Discolouration. On rare occasions, specific conditions arise that allow copper to be leached out of pipes into the drinking water. This is not typically a hazard since copper in trace amounts is an essential nutrient. However, in older homes, lead may have been used in some of the pipes. The same conditions that can leach copper can also leach lead, which is very toxic and not easily detected.
If you suspect your home may be of the age where lead pipes were used during construction, you may want to contact your regional health authority to ask them to test your water. |
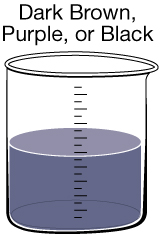 |
Manganese Discolouration. Much like iron, manganese can naturally enter drinking water from soil and rocks. In addition to tinting the water, it can sometimes be found as small black specks in a glass of water. Manganese is an essential nutrient, and in small qualities it does not have any negative health effects. |
Taste and Odour
Some of the common problems experienced with the taste and smell of drinking water are listed in the following table.
Water Taste and Odour |
Common Cause |
chlorine |
Chlorine. Chlorination is used in the water-treatment process. |
rotten egg |
Hydrogen Sulfide Gas. This can occur in cases where tap water is coming from a well. Decomposing deposits of underground organic matter produce this gas, which may then enter the well water. Hydrogen sulfide gas can also enter well water that is near coal deposits or oil fields.
In some cases, the water itself is not the cause of the rotten-egg smell; rather it is coming from the drain. Anaerobic bacteria can thrive in this environment, producing hydrogen sulfide gas that accumulates underneath the food trap. When the water is turned on, the gas is forced out of the drain, yielding the rotten-egg smell. This problem can be fixed by pouring a cup of household bleach down the drain to kill the bacteria.
It is also possible that the smell is coming from the hot water heater, which has a magnesium rod that can interact with sulfates in the water to produce hydrogen sulfide gas. This is likely the problem if the rotten-egg smell is present in hot water but not cold water.
Hydrogen sulfide gas is not harmful at the levels you would find in tap water, and the bacteria that produce it pose no known health risk. However, if the smell is indeed coming from the water and has appeared for no previously known reason, it would be a good idea to call your water supplier to inform them of the problem. Hydrogen sulfide gas can, in rare cases, result from pollution or sewage contamination. |
fishy or swampy |
Algae Growth. There are many different tastes and odours that are the result of algae growth in reservoirs. Depending on the type of algae, the water can taste and smell fishy, swampy, grassy, and even like cucumber! There are no health risks associated with smells related to algae growth. |
 Self-Check
Self-Check
SC 1. A number of substances, in addition to their possible classification, are listed in the following table. Recall that pure substances may be classified as an element or a compound. Mixtures may be classified as homogeneous or heterogeneous. Fill in this table. The first row is done for you.
Substance |
Pure Substance |
Element |
Homogeneous Mixture |
distilled water |
pure substance |
compound |
-------- |
smoke-filled air |
|
|
|
24-karat gold ring |
|
|
|
grape juice from concentrate |
|
|
|
salty water |
|
|
|
milk |
|
|
|
sugar |
|
|
|
18-karat gold ring |
|
|
|
freshly squeezed orange juice |
|
|
|
blood |
|
|
|
methanol, CH3OH(l) |
|
|
|
molten lead |
|
|
|
SC 2. Some substances may form a homogeneous mixture or a heterogeneous mixture, depending on environmental conditions. For each of the following, explain when it would be classified as a homogeneous mixture and when it would be classified as a heterogeneous mixture.
- humid air
- soda pop
- ice cubes
 Self-Check Answers
Self-Check Answers
SC 1.
Substance |
Pure Substance |
Element |
Homogeneous Mixture |
distilled water |
pure substance |
compound |
-------- |
smoke-filled air |
mixture |
------- |
heterogeneous mixture |
24-karat gold ring |
pure substance |
element |
------- |
grape juice from concentrate |
mixture |
------- |
homogeneous mixture |
salty water |
mixture |
------- |
homogeneous mixture |
milk |
mixture |
------- |
heterogeneous mixture |
sugar |
pure substance |
compound |
------- |
18-karat gold ring |
mixture |
------- |
homogeneous mixture |
freshly squeezed orange juice |
mixture |
------- |
heterogeneous mixture |
blood |
mixture |
------ |
heterogeneous mixture |
methanol, CH3OH(l) |
pure substance |
compound |
------- |
molten lead |
pure substance |
element |
------- |
SC 2.
- Humid air is normally a homogeneous mixture of air and water vapour; but if the air becomes so humid that fog or precipitation forms, it becomes a heterogeneous mixture because of the visible liquid water droplets that form.
- When soda pop is in a sealed bottle, it is a homogeneous mixture of the syrup solution and dissolved carbon dioxide. If the pressure is reduced, such as when you open the bottle and pour it into a glass, carbon dioxide gas bubbles form in the pop, making the solution heterogeneous.
- Tap water contains both solid and gas impurities. As water freezes, these impurities are pushed towards the centre of the ice cube. This process accounts for the cloudy centre of an ice cube. The presence of many small oxygen bubbles makes the ice cube heterogeneous. If the water is boiled for several minutes, all of the gases in the water will escape. This will result in a clear homogeneous ice cube.