Module 4 Intro
1. Module 4 Intro
1.21. Page 4
Module 4—Properties of Solutions
 Self-Check
Self-Check
SC 1. Use the kinetic molecular theory to explain why solids and gases have opposite solubility characteristics.
SC 2. The solubility of sucrose is 180 g/100 mL at 0 °C. How much sugar is dissolved in the following situations?
- 170 g is placed in 100 mL of water.
- 180 g is placed in 100 mL of water.
- 190 g is placed in 100 mL of water.
- 600 g is placed in 300 mL of water.
SC 3. The solubility of nitrogen gas is 0.003 g/100 mL at STP conditions. How much nitrogen gas is dissolved in the following situations?
- 0.002 g is bubbled in 100 mL of water.
- 0.003 g is bubbled in 100 mL of water.
- 0.004 g is bubbled in 100 mL of water.
- 0.010 g is bubbled in 400 mL of water.
SC 4. Explain the difference between the terms solubility and saturated solution.
SC 5. If you have a solution of table salt dissolved in water, describe three ways you could turn this into a saturated solution.
SC 6. Give two reasons why stains are easier to remove in warm water than in cold water.
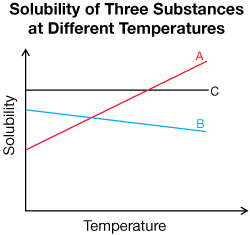
SC 7. In the following graph, which substance is likely a gas?
 Self-Check Answers
Self-Check Answers
SC 1. Solids dissolve better in warm solutions because the water molecules have more energy to break bonds holding the solid together. Gases dissolve worse in warm solutions because the gas molecules have more energy and move faster, escaping the solution.
SC 2.
- All 170 g will dissolve.
- All 180 g will dissolve.
- Only 180 g will dissolve. 10 g will remain undissolved.
- First, determine the maximum amount of solute that can dissolve. If 100 mL can hold 180 g, then 300 mL can hold 540 g. If 600 g of sucrose is placed in the solution, only 540 g will dissolve. The other 60 g will remain undissolved.
SC 3.
- All 0.002 g will dissolve.
- All 0.003 g will dissolve.
- Only 0.003 g will dissolve. 0.001 g will escape the solution.
- First, determine the maximum amount of solute that can dissolve. If 100 mL can hold 0.003 g, then 400 mL can hold 0.012 g. If 0.010 g of nitrogen is placed in the solution, all of it will dissolve.
SC 4. The term solubility refers quantitatively to the maximum amount of solute that can be dissolved in water. It can be expressed as a number with units of g/mL (or mol/L). A saturated solution is a qualitative term describing a solution that will not dissolve any more solute at a given temperature.
The two terms may be used in a sentence as follows:
A saturated solution of sucrose has a solubility of 487 g/100 mL at 100 ºC.
SC 5. Three ways you could turn this solution into a saturated solution are as follows:
- You could add more NaCl(s) until the solution is saturated.
- You could cool the solution to reduce the solubility.
- You could allow water to evaporate from the solution.
SC 6. At warmer temperatures, both the detergent and stain substances will have a higher solubility in water. Also, with the increase in temperature, the rate of chemical reactions increases.
SC 7. Substance B is likely a gas because the solubility decreases as temperature increases.