Module 4 Intro
1. Module 4 Intro
1.24. Page 7
Module 4—Properties of Solutions
 Read
Read
Dynamic Equilibrium
At the beginning of this lesson you completed a Try This exercise where you made observations and wrote explanations about what was occurring within a saturated solution.
dynamic equilibrium: the idea that there is a balance between two opposing processes (forward and reverse) occurring at the same rate
When the solution was cooled, you observed that salt crystals were seen on the bottom of the beaker. While it may appear the salt crystals are no longer dissolving, they actually are. The amount of solid appears to remain constant because even though some of the solid is dissolving, an equal amount of solid is crystallizing out of the solution. This process is called dynamic equilibrium.
Read the section “Explaining Saturated Solutions” on page on 224 in your textbook. Pay close attention to the description of the experimental evidence that was used to test the theory of dynamic equilibrium described on pages 225 and 226.
How does the figure below demonstrate the dynamic equilibrium in the saturated solution of salt water you observed earlier in this lesson?
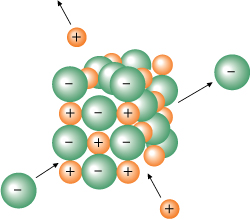
In the dynamic equilibrium involving a salt crystal in a saturated solution, there is still dissociating, but the ions that leave are immediately replaced by other ions that enter the solid from the solution. This process is similar to having a party for 20 people—if two new guests arrive and two people leave, the total number of people present at the party remains the same.
 Self-Check
Self-Check
SC 11. State two conditions required for a solution to be in dynamic equilibrium.
SC 12. While salt is being poured into a beaker that initially contains distilled water, would the system be in a state of dynamic equilibrium?
SC 13. Explain what is happening to a salt crystal in a dynamic equilibrium system.
SC 14. Describe an experimental technique to test for dynamic equilibrium in a saturated solution of strontium chloride.
 Self-Check Answers
Self-Check Answers
SC 11. Two opposing processes must be occurring at exactly the same rate. The solution must be in a closed system (the volume of water is constant, the amount of solute is constant, the temperature is constant, etc.).
SC 12. No. Dynamic equilibrium means two opposing processes must be occurring at exactly the same rate. When the salt is being poured in, it is dissolving at a far greater rate than it is crystallizing.
SC 13. A salt crystal is both dissolving and crystallizing at the same rate. As such, the mass of the crystal remains constant, even though it is constantly being broken and reformed.
SC 14. Create a saturated solution of strontium chloride with some undissolved solute visible at the bottom. Add a few drops of radioactive strontium chloride; then measure where the radiation is coming from within the solution. After time passes, if radiation is coming from within the solid, that means the radioactive sample has crystallized into the solid.