Module 6 Intro
1. Module 6 Intro
1.7. Page 5
Module 6—Stoichiometry
 Discuss
Discuss
Construct a two-column table. In the first column, in separate rows, list each of the four assumptions about written chemical reactions. In the second column, list examples of each assumption that you can identify from your experiences in this course and in other science courses.
Place a copy of your table into your course folder, and then post a copy to the discussion board. Read the postings of two other students and comment on the suitability of the examples they have used. You may wish to modify your table based on the examples and feedback you get from other students. Save any revised copies of your table in your course folder. Share your table with your teacher.
 Read
Read
net ionic equation: a type of balanced chemical equation that lists only the reacting particles
In Module 4, Lesson 1, you learned the effect water can have on dissolving ionic compounds. The process of dissociation creates separated ions, each with the possibility to collide with other ions and participate in a chemical reaction. To communicate which ions are participating in a chemical reaction, a unique type of balanced chemical equation is used—the net ionic equation. A net ionic equation is a type of balanced chemical equation that lists only the reacting particles.
Read “Net Ionic Equations” on pages 281 to 283 of your textbook. Work through the sample problem and examples.
 Self-Check
Self-Check
SC 7. Complete “Practice” questions 10, 11, and 13 on page 284 of your textbook.
 Self-Check Answers
Self-Check Answers
SC 7. “Practice” questions 10, 11, and 13, page 284
-
Write the balanced chemical equation.
Pb(s) + 2 AgNO3(aq) → Pb(NO3)2(aq) + 2 Ag(s)
Now, write the total ionic equation.
Pb(s) + 2 Ag+(aq) + 2 NO3–(aq) → Pb2+(aq) + 2 NO3–(aq) + 2 Ag(s)
Cancel the ions that appear on both sides of the equation.
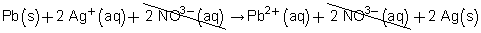
Therefore, the net ionic equation is
Pb(s) + 2 Ag+(aq) → Pb2+(aq) + 2 Ag(s)
- (a)
Balanced chemical equation: 3 CaCl2(aq) + 2 Na3PO4(aq) → Ca3(PO4)2(s) + 6 NaCl(aq)
Total ionic equation: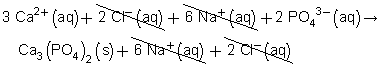
Net ionic equation: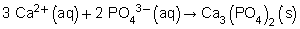
(b) The spectator ions are chloride and sodium.
-
(a)
Balanced chemical equation: HNO3(aq) + NaHCO3(aq) → NaNO3(aq) + H2CO3(aq)
Total ionic equation: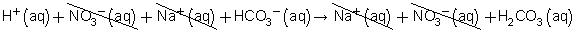
Net ionic equation: H+(aq) + HCO3−(aq) → H2CO3(aq)
(b) The spectator ions are nitrate and sodium.
 Module 6: Lesson 1 Assignment
Module 6: Lesson 1 Assignment
Complete the Module 6: Lesson 1 Assignment that you saved to your course folder at the beginning of this lesson. Submit your assignment to your teacher.