Module 7 Intro
Completion requirements
Created by IMSreader
1. Module 7 Intro
1.8. Page 6
Module 7—Chemical Analysis
 Lesson Summary
Lesson Summary

Courtesy NASA
In this lesson you explored this essential question:
-
How can limiting reagents be used to predict the amount of product for a chemical process?
Developing a “quick fix” to control the CO2(g) concentration in the Apollo 13 spacecraft demonstrates what must be considered when chemical systems have a limited amount of one of the reactants.
As part of your Module Assessment you used stoichiometry to calculate the mass of lithium hydroxide necessary to remove excess carbon dioxide from the air inside a spacecraft on an Apollo mission. In a situation such as space travel, making careful calculations involving necessary components is essential to ensure the safety and success of the mission.
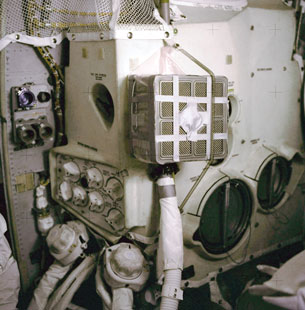
Courtesy NASA