Module 1
1. Module 1
1.11. Page 4
Module 1—Thinking Energy
 Going Beyond
Going Beyond
Calorimetric data about the energy content in foods is not always reliable. Use the Internet to research other methods used to determine the energy content in foods. Why are other methods used? Do the other methods have limitations or do they provide greater accuracy than calorimetric data?
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1 to 8 on page 487 in the textbook.
 Self-Check Answer
Self-Check Answer
SC 1.
- Energy cannot be created nor destroyed.
- All energy comes from some other form of energy.
Practice 3.
Practice 4.
- If the mass of material undergoing temperature change is doubled and the other variables remain the same, then the thermal energy is doubled.
- If the specific heat capacity of material undergoing temperature change is divided by two (is halved) and the other variables remain the same, then the thermal energy is halved.
- If the temperature change of the material is tripled and the other variables remain the same, then the thermal energy is tripled.
- If all three variables (m, c, and Δt) are doubled, then the thermal energy is increased by a factor of 8 (2 × 2 × 2 = 8).
Practice 6.
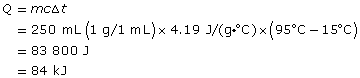
The gain in the water’s thermal energy is 84 kJ.
Practice 7.
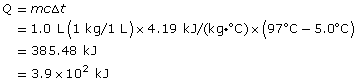
The gain in the water’s thermal energy is 3.9 x 102 kJ.
Practice 8.
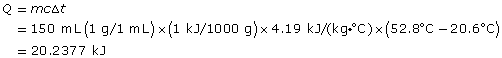
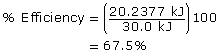
The calorimeter is 67.5% efficient.
Evaluate the Design of a Solar Panel
 Watch and Listen
Watch and Listen

Natural Resources Canada
Drake Landing is an innovative neighborhood located in Okotoks, Alberta. Part of Drake Landing’s innovation involves the way the development uses solar energy to greatly reduce the neighbourhood’s dependence on fossil fuels. As shown in the picture, energy from the sun is used to heat homes. This greatly reduces the need for fossil fuels.
View the video “Drake Landing Solar Community” for more information on how a community solar heating system works.
The solar panels found on the roofs of buildings within the Drake Landing Solar Community are part of an active solar heating system. The circulation of antifreeze solution through the system allows for the transfer of energy from the collector to the house or to the borehole energy storage system.
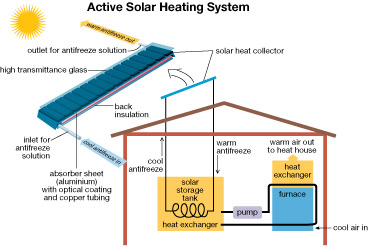
This is a schematic representation of a solar heating system and its components.
 Module 1: Lesson 2 Assignment
Module 1: Lesson 2 Assignment
Retrieve your copy of the Module 1: Lesson 2 Assignment that you saved to your computer earlier in this lesson. You will use your knowledge of energy transfers and of how an energy-measuring device such as a calorimeter works. Complete Part 2—Evaluate the Design of a Solar Collector, and save your work in your course folder. You will receive information later in the lesson on when to submit your work to your teacher.
 Reflect on the Big Picture
Reflect on the Big Picture

© 2000-2007, It’s Our World Recycling. Adapted with permission. <www.iowr.ca>
As you work through Module 1, you are developing a plan for an ecotour. You have been saving related work to your ecotour planning sub-folder. Ecotourism is defined as “the responsible travel to natural areas that conserves the environment and improves the well-being of local people” (The International Ecotourism Society). At the start of Lesson 2 you investigated the energy content of different foods. What foods should the participants in your ecotour eat to maintain their energy and nutrition levels?
Choice of Snack Foods
RBP 1. Prepare a list of snack foods that you intend to provide during the ecotour you are planning. Include the energy content of each food, and justify the food's use. Identify what you need to consider when selecting and using these products, and show how your use of these foods supports the principles of ecotourism. Place a copy of your list and supporting information in your ecotour planning sub-folder.
This is the first of six assignments that you can submit as part of your Module 1 Assessment. For more information, refer to the Module 1 Assessment.
 Module 1: Lesson 2 Assignment
Module 1: Lesson 2 Assignment
Submit your completed Module 1: Lesson 2 Assignment to your teacher.