Module 1
1. Module 1
1.29. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
In Lesson 5, you learned to calculate the enthalpy of a reaction per mole of one of the process' reactants or products. Communicating a reaction enthalpy in this way allows specific information to be expressed.
Enthalpy changes are communicated in four ways:
- molar enthalpy of reaction for a specific reactant
- enthalpy change for a balanced reaction equation
- inclusion of the energy as a term in a balanced reaction equation
- chemical potential energy diagrams
Read the text and work through the “Sample problems” and the “Communication examples” on pages 495–499 in the textbook. Complete TR 1 as you read.
 Try This
Try This
TR 1. Complete a table like the following in your course folder to summarize the four methods as you read about them:
Question |
Molar enthalpy of reaction for a specific reactant |
Enthalpy change for a balanced reaction equation | Inclusion of the energy as a term in a balanced reaction equation | Chemical potential energy diagrams |
How is this method done? |
||||
What do I need to remember to do this method correctly? |
TR 2. Test your understanding and your summaries by attempting “Communication example 4” on page 500 in the textbook.
![]() Post your table in the discussion area for your class and read the summaries of at least two other students. If necessary, revise your table. Save a copy of your revised table in your course folder.
Post your table in the discussion area for your class and read the summaries of at least two other students. If necessary, revise your table. Save a copy of your revised table in your course folder.
 Self-Check
Self-Check
SC 1. Complete “Section 11.3” questions 1, 2, 3, 4, 5, and 7 on page 501 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Section 11.3 1.
- The symbols used represent the following: Δ = change, c = combustion, H = enthalpy, m = molar, º = standard conditions. Therefore, the symbols represent the molar enthalpy change for a combustion reaction (e.g., methane at standard conditions).
- The symbols used represent the following: n = amount, Δ = change, H = enthalpy, m = molar, º = standard conditions. Therefore, the symbols represent the moles of one substance (e.g., propane) involved in a chemical process multiplied by its molar enthalpy of reaction (e.g., molar heat of combustion for propane).
- The symbol used represents the thermal energy change for water.
Section 11.3 2.
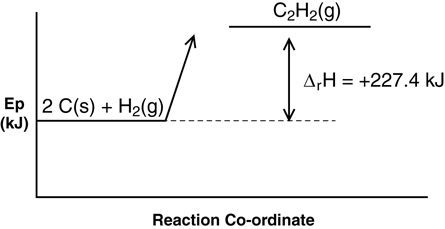
- ΔrHmº = +227.4 kJ/mol
2 C(s) + H2(g) → C2H2(g) ΔrHº = +227.4 kJ
2 C(s) + H2(g) + 227.4 kJ → C2H2(g)
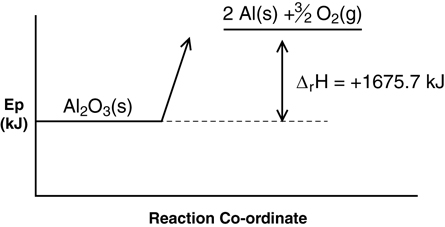
- ΔrHmº = +1675.7 kJ/mol
Al2O3(s) → 2 Al(s) + 3/2 O2(g) ΔrHº = +1675.7 kJ
Al2O3(s) + 1675.7 kJ → 2 Al(s) + 3/2 O2(g)
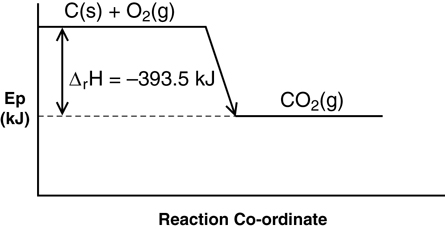
- ΔrHmº = -393.5 kJ/mol
C(s) + O2(g) → CO2(g) ΔrHº = -393.5 kJ
C(s) + O2(g) → CO2(g) + 393.5 kJ
Section 11.3 3.
- ΔrHmº = -241.8 kJ/mol of hydrogen
- ΔrHmº = -318.0 kJ/mol of ammonia
- ΔrHmº = +81.6 kJ/mol of nitrogen
- ΔrHmº = -372.8 kJ/mol of iron
Section 11.3 4.
- ΔrH = -114 kJ
- H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 H2O(l) ΔrH = -114 kJ
- ΔrH = -114 kJ / 1 mol = -114 kJ/mol of sulfuric acid
- ΔrH = -114 kJ / 2 mol = -57 kJ/mol of aqueous sodium hydroxide
Section 11.3 5.
- CH3OH(l) + 3/2 O2(g) → CO2(g) + 2 H2O(g) + ΔcH = -725.9 kJ
- C(s) + ¼ S8(s) → CS2(l) ΔfH = +89.0 kJ
- ZnS(s) + 3/2 O2(g) → ZnO(s) + SO2(g) ΔcH = -441.3 kJ
- Fe2O3(s) → 2 Fe(s) + 3/2 O2(g) ΔsdH = +824.2 kJ
Section 11.3 7.
- H2(g) + ½ O2(g) → H2O(g) ΔrH = -285.8 kJ
H2O(g) → H2(g) + ½ O2(g) ΔrH = +285.8 kJ
- The magnitude of the enthalpy changes are identical, except that the combustion reaction is exothermic and the decomposition reaction is endothermic.
ΔrH(combustion) = -ΔrH(decomposition)