Module 1
1. Module 1
1.33. Page 2
Module 1—Thinking Energy
 Explore
Explore
 Read
Read
In Lesson 2 you used a calorimeter to measure the enthalpy of a reaction. You also learned that by using a bomb calorimeter you can improve the reliability and range of reactions that you can investigate. However, is it possible to measure the enthalpy change of every reaction using a calorimeter? Is it possible to use your understanding of chemical potential energy to explain why energy changes occur during chemical reactions? Have you considered how this understanding might be used to determine the enthalpy change for a process if it cannot be done experimentally?
Read pages 502–503 in the textbook to learn about Hess’ law.
 Self-Check
Self-Check
SC 1. Identify two instances in which it is difficult to determine the energy change for a reaction experimentally using equipment normally found in a high school laboratory.
SC 2. Sketch “Figure 2” on page 503 of the textbook. Add details to your sketch to
- identify the separate reactions (steps) in the process
- explain why the overall enthalpy change is only -110.5 kJ, not -393.5 kJ
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1. It may not be possible to determine the energy change for a reaction experimentally if the reaction occurs too slowly, if equipment is unavailable, or if there are safety concerns.
SC 2.
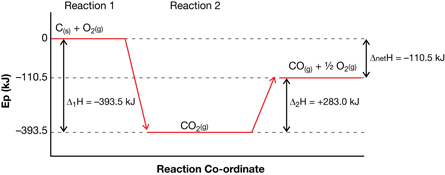
The overall change is -110.5 kJ, which represents the difference between the initial and final potential energies of the reactants and the final product. The sum of the reaction enthalpies is -110.5 kJ.
 Try This
Try This
Use your answers to the Self-Check questions and the information in your textbook to demonstrate your understanding of Hess' law by answering the following questions.
Consider the following information about a theoretical chemical system:
Reaction 1: AB → A + B ΔrH = +75 kJ
Reaction 2: A + CD → AC + D ΔrH = -50 kJ
Reaction 3: BD → B + D ΔrH = -125 kJ
Net Reaction: AB + CD → AC + BD
TR 1. Demonstrate how reactions 1–3 can be combined to yield the net reaction.
TR 2. Demonstrate how reactions 1–3 can be depicted on a potential energy diagram such that the enthalpy change for the net reaction is depicted.
TR 3. Demonstrate how the enthalpy change for reactions 1–3 are combined to result in an enthalpy change for the net reaction.
Submit your responses to your teacher and save a copy in your course folder. If your teacher provides feedback, carefully review his or her feedback and revise your answer, if necessary.
![]() Post your responses to TR 1–3 in the discussion area for your class. Review the responses of at least two other students. Identify where your responses were similar to and different from your classmates' responses. If possible, evaluate the different approaches used by your classmates, and determine whether you might use parts of their strategies. If necessary, revise your answers to TR 1–3 and save a copy of your revised responses in your course folder.
Post your responses to TR 1–3 in the discussion area for your class. Review the responses of at least two other students. Identify where your responses were similar to and different from your classmates' responses. If possible, evaluate the different approaches used by your classmates, and determine whether you might use parts of their strategies. If necessary, revise your answers to TR 1–3 and save a copy of your revised responses in your course folder.
 Read
Read
Work through “Sample problem 11.4” and “Communication example” on pages 503–504 in the textbook.
![]() You may go to the website for your textbook to listen to the explanation of “Sample problem 11.4.” Contact your teacher for instructions on how to access this website.
You may go to the website for your textbook to listen to the explanation of “Sample problem 11.4.” Contact your teacher for instructions on how to access this website.
Review the “Summary” on page 504 of the textbook for clarification of the process used to solve these problems.
 Self-Check
Self-Check
SC 3. Complete “Practice” questions 1–4 on pages 504–505 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
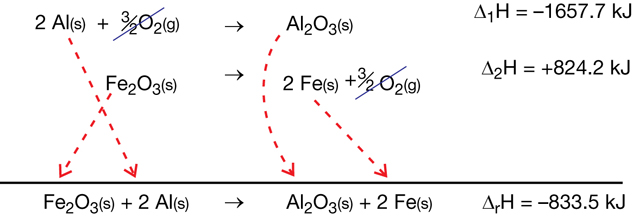
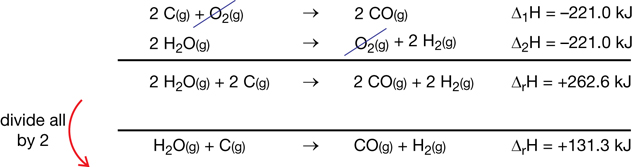
SC 3.
Practice 1.
Practice 2.
Practice 3.
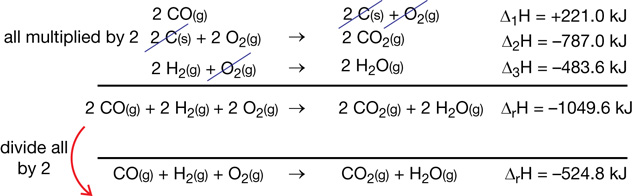
Practice 4.
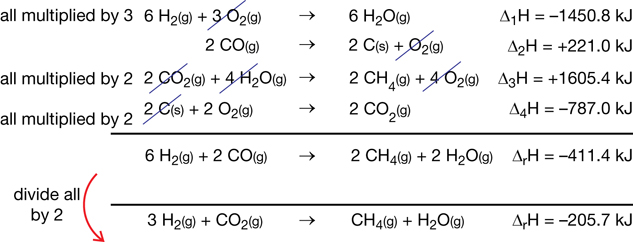
 Try This
Try This
To some people, the most important rules for Hess’ law are as follows:
- If the chemical reaction is reversed, then the sign of the change in enthalpy is reversed.
- If the coefficients of the chemical reaction are altered, then the change in enthalpy is also altered by the same factor.
It is easy to memorize these rules, but it is important to do more than memorize them—you must understand them.
TR 4. Write explanations that identify the scientific principles associated with each of these rules and state why these rules need to be considered when using Hess’ law.
Save a copy of your response in your course folder.
![]() Post a copy of your response to the discussion area for your class. Read the responses of at least two other students. Provide feedback to your classmates to help them ensure their explanations are clear and thorough. Revise your explanations based on the feedback you receive. Save a copy of your revised explanations in your course folder.
Post a copy of your response to the discussion area for your class. Read the responses of at least two other students. Provide feedback to your classmates to help them ensure their explanations are clear and thorough. Revise your explanations based on the feedback you receive. Save a copy of your revised explanations in your course folder.