Module 8
1. Module 8
1.13. Page 2
Module 8—Acid-Base Equilibrium
 Explore
Explore
At least one of the summaries you started in Lesson 1 will have covered early theories for acids and bases. The early theories proposed that dissociation of solutes to produce hydrogen ions or hydroxide ions could explain the acidic and basic properties of solutions. A flaw of these theories was the lack of an ability to explain the properties of common substances that clearly have acidic or basic properties.
- A modification to these early theories required the consideration of the possibility of a chemical reaction between the solute and water. For example, the reaction between aqueous hydrogen chloride, HCl(aq), with water is
- HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq)
The product of this reaction is the hydronium ion; it was later confirmed to be the acidic particle.
- The reaction of a substance like ammonia, NH3, with water is
- NH3(aq) + H2O(l) → NH4+(aq) + OH–(aq)
The earlier theory could not explain the basic properties of aqueous ammonia, but the reaction with water shows the probability of hydroxide ions being produced as products of the reaction. Simple diagnostic tests, like litmus, confirm this theoretical explanation.
 Discuss
Discuss
In previous lessons you considered each reaction as an equilibrium, and you used methods to communicate equilibrium position. Consider the following systems:
System |
HCl(aq) + H2O(l) → H3O+(aq) + Cl–(aq) |
Concentration of HCl(aq) |
0.0250 mol/L |
Solution pH |
1.6 |
System |
NH3(aq) + H2O(l) → NH4+(aq) + OH–(aq) |
Concentration of NH3(aq) |
0.0250 mol/L |
Solution pH |
10.8 |
D 1. Explain how you would use the information provided to determine the equilibrium positions for the two systems. Give reasons for your suggested approach.
Post your proposed strategy to the discussion area for your class. Read the strategies of at least two other students. How are the other students using the data provided? Will their strategies work? Provide constructive feedback to your classmates regarding the strategies they posted. Revise your strategy based on your readings and the feedback you receive. Make sure you record the reason for any modifications made. Save your work in your course folder.
D 2. Use the information provided to determine and appropriately communicate the equilibrium position for the reactions shown.
Save a copy of your answers in your course folder and submit a copy to your teacher.
 Read
Read
You might conclude that it appears that many substances require a reaction with water to exhibit their properties. Do any general trends exist in these reactions?
Read pages 722–724 in the textbook to examine the trends that might exist in the reactions of acids and bases.
Brønsted-Lowry Concept
In the textbook reading you learned that the Brønsted-Lowry concept redefines how you will consider acids and bases as entities in a chemical reaction. According to this theory you will describe acids and bases as a result of the kind of chemical change they undergo, rather than by their empirical properties.
Because you have seen that some substances have the ability to react as either an acid or a base, you will further modify your concept of acids and bases. Later in this module you will learn how to predict when these substances will act in one of these possible roles.
 Self-Check
Self-Check
SC 1. Complete “Practice” questions 1–5 on page 724 of the textbook.
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 1.
Practice 1.
- According to the Arrhenius theory, acids are substances that ionize in water to form aqueous hydrogen ions.
- According to the modified Arrhenius theory, acids are substances that react with water to form aqueous hydronium ions.
- According to the Brønsted-Lowry concept, acids are substances that donate a hydrogen ion (proton) during chemical reactions.
Practice 2.
Both definitions predict the hydroxide ions as a product of the processes involved but differ in explaining how the hydroxide ion is produced. The Arrhenius theory explains the ion's production as a result of dissociation of the solute. The Brønsted-Lowry concept requires that a substance react with water; in the resulting transfer of a proton from the water molecule to the solute, a hydroxide ion is produced.
Practice 3.
- HF(aq) acid, SO32–(aq) base
- CO32–(aq) base, CH3COOH(aq) acid
- H3PO4(aq) acid, OCl–(aq) base
- HCO3–(aq) base, HSO4–(aq) acid
Practice 4.
- HSO3–(aq) + OH–(aq)
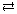 SO32–(aq) + H2O(l)
SO32–(aq) + H2O(l)
acid base
- HSO3–(aq) + H3O+(aq)
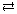 H2SO3(aq) + H2O(l)
H2SO3(aq) + H2O(l)
base acid
Practice 5.
The Brønsted-Lowry concept allows for the ability to describe the reaction between acids and bases and to explain how some substances could behave as amphiprotic species.