Module 3
1. Module 3
1.17. Lesson 3
Module 3—Electrical Phenomena
Lesson 3—Applying Coulomb’s Law
 Get Focused
Get Focused

Ernie Blair, Huntsville-Madison Co. (AL) 9-1-1 Center
This photograph was taken the day after a thunderstorm. Lightning struck the pole and then followed the guy wire down into the sandy soil. Closer inspection reveals one of the effects of the lightning—some of the sand was turned into glass.

Ernie Blair, Huntsville-Madison Co. (AL) 9-1-1 Center
When lightning strikes the sandy soil, the extremely high temperature causes the silica in the sand to melt. The rapid cooling of this molten material forms glass. The next photograph shows a close-up of white silica glass formed by a lightning strike. Note the grains of sand melded to the outside surface of the glass.
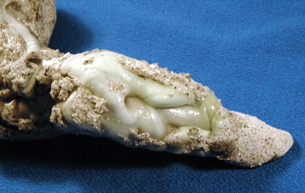
Photo by Stan Celestian, Glendale Community College Arizona.
Although the white glass has a smooth appearance, on the atomic scale, the silica in the glass has formed crystals with the main building block being a pyramid shape. In the following ball-and-stick model, the white ball in the centre represents a silicon atom; the four red balls surrounding it represent oxygen atoms. The oxygen atoms are actually much larger than the silicon atom, but they have been reduced in size to make the detail of the inner structure more visible.
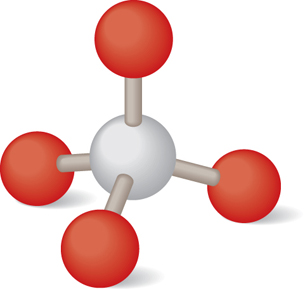
Each bond forms because of an unequal sharing of electrons. Each oxygen atom has a slightly negative charge, and the central silicon atom has a slightly positive charge. The result is a tetrahedral shape that is perfectly symmetrical: the values for the angles and the distances of separation are the same for each oxygen-silicon bond. Why does nature produce this kind of symmetry? The answer has to do with Coulomb’s law.
In this lesson you will apply Coulomb’s law to the exploration of both large-scale and extremely small-scale phenomena:
- How much charge is transferred in a lightning strike, and how is this amount of charge measured?
- How can Coulomb’s law be applied to predict the net force acting on one point charge due to the presence of other point charges? How does this sort of analysis relate to the symmetry found in crystals?
 Module 3: Lesson 3 Assignments
Module 3: Lesson 3 Assignments
Your teacher-marked Module 3: Lesson 3 Assignment requires you to submit a response to the following questions:
- Assignment—A 1, A 2, A 3, and A 4
You must decide what to do with the questions that are not marked by the teacher.
Remember that these questions provide you with the practice and feedback that you need to successfully complete this course. You should respond to all the questions and place those answers in your course folder.