Module 6
1. Module 6
1.8. Page 2
Module 6—Wave-Particle Duality and Quantum Physics
 Explore
Explore
 Read—The Photoelectric Effect
Read—The Photoelectric Effect
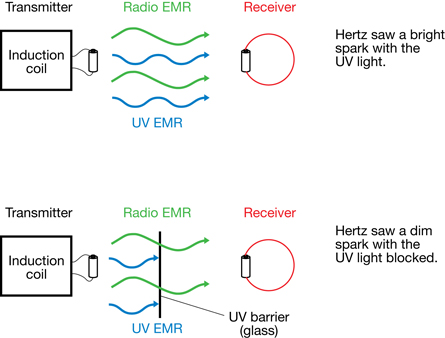
In 1887 during his investigations into Maxwell's Theory of Electromagnetic Waves (Module 5: Lesson 1), Heinrich Hertz discovered the photoelectric effect. As shown in the diagram above, Hertz found that the ultraviolet light produced by the spark in his radio transmitter greatly enhanced the spark that was observed at the receiver. This provided indirect evidence that the electrons in some metals are ejected when light of sufficient frequency contacts it. In Hertz’s experiment, the ultraviolet light produced by the spark at the transmitter supported the production of a spark at the receiver by ejecting electrons from its metal surface. When the ultraviolet light was blocked from reaching the receiver, the intensity of the sparks at the receiver was noticeably reduced.
The photoelectric effect is based on the fact that certain metallic surfaces lose their negative charges when exposed to ultraviolet light. Consider the apparatus illustrated below. When ultraviolet light is incident on a polished zinc surface, the negatively charged leaves of the electroscope fall. This indicates that the electroscope loses negative charge when ultraviolet light makes contact with the polished metal surface.
photoelectric effect: the emission of electrons when a metal is illuminated by EMR with a frequency greater than or equal to the metal’s threshold frequency
photoelectron: an electron emitted from a metal because of the photoelectric effect
threshold frequency: the minimum frequency that a photon can have to cause photoemission from a metal
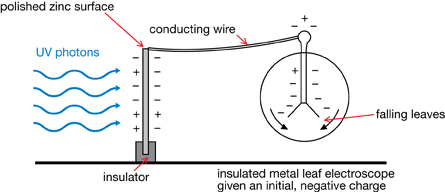
If the zinc plate is replaced with a non-metal surface such as glass, the electroscope is unaffected indicating that the photoelectric effect does not occur on the surface of insulators.
It was found that a few elements, namely the alkali metals (lithium, sodium, potassium, rubidium, and cesium), will eject electrons, commonly referred to as photoelectrons, when visible or UV light is incident on them. For this reason the alkali metals are commonly used in photoelectric cells and other devices that make use of the photoelectric effect. Metals from outside the alkali group will also eject photoelectrons but generally require higher frequency, higher energy EMR beyond the visible spectrum (UV, X-ray, and Gamma rays).
The following general observations have been made with regard to the photoelectric effect:
- Electrons are emitted instantly when EMR is incident on the surface. There is no time required for the electrons in the metal to build up energy before escaping.
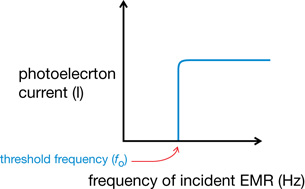
- A threshold frequency of EMR is required to cause the emission of photoelectrons. If the light shining on the photoelectric surface is below a certain frequency, there is no photoelectron emission regardless of the intensity, or brightness, of the light. If the frequency is higher than the threshold frequency, photoelectron emission occurs regardless of the intensity. This is the minimum frequency of EMR that causes the photoelectric effect for a specific metal.
- According to quantum theory, the intensity, or brightness, of the incident light indicates the number of photons being emitted. Therefore, bombarding a metal with many photons at a frequency lower than the threshold has no effect.
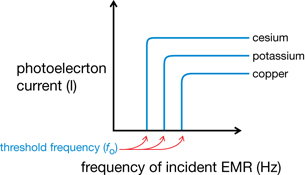
- Each type of metal has its own characteristic threshold frequency.
- When the frequency of the incident EMR increases beyond the threshold frequency, the kinetic energy of the released photoelectrons shows a corresponding increase. (A photoelectron is a regular electron ejected from a metal during the photoelectric effect.)
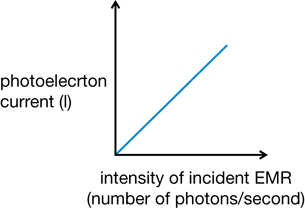
- If the light is at or above the threshold frequency, increasing the intensity will increase the number of photoelectrons, but not the energy of any individual photoelectron. The current (C/s) would increase because more electrons are released each second. This shows that the number of photoelectrons is proportional to the number of photons.
These general observations can be illustrated graphically.
Graph 1: Current vs. Intensity of Incident EMR
Graph 2: Current vs. Frequency of Incident EMR
Graph 3: Current vs. Frequency of Incident EMR for Various Metals
Read “The Photoelectric Effect” on pages 711 and 712 of your physics textbook for more information.