Module 6
1. Module 6
1.11. Page 5
Module 6—Wave-Particle Duality and Quantum Physics
 Lesson Summary
Lesson Summary
You explored the following questions in this lesson:
-
What is the photoelectric effect?
-
How is the photoelectric effect described by quantum theory?
-
How does the photoelectric effect support the notion of wave-particle duality?
In this lesson you learned that the photoelectric effect is characterized by the following general observations:
-
Electrons are emitted instantly when EMR is incident on the surface.
-
A threshold frequency of EMR is required to cause the emission of photoelectrons. If the light shining on the photoelectric surface is below a certain frequency, there is no photoelectron emission, regardless of the intensity, or brightness, of the light. If the frequency is higher than the threshold frequency, photoelectron emission occurs regardless of the intensity.
-
Each type of metal has its own characteristic threshold frequency and when the frequency of the incident EMR increases beyond the threshold frequency, the kinetic energy of the released photoelectrons shows a corresponding increase.
-
If the light is at or above the threshold frequency, increasing the intensity will increase the number of photoelectrons, but not the energy of any individual photoelectron.
![]()
These relationships were verified by Millikan’s experiments using stopping voltage to measure accurately the kinetic energy of the photoelectrons.
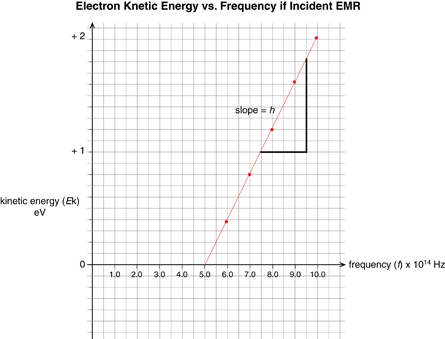
The photoelectric effect can also be illustrated graphically by plotting the photoelectron kinetic energy versus the frequency of the incident light. When this is done, the graph forms a straight line defined by y = mx + b, which means, according to the mathematical definition of the photoelectric effect, the slope of the line is equal to Planck’s constant and the x-intercept is equal to the threshold frequency of the metal.
Einstein’s explanation of the photoelectric effect treated EMR as if it had particle characteristics in terms of light quanta. Doing so helped scientists understand the photoelectric effect. In summary, the photoelectric effect, in combination with the wave-like characteristics of EMR from other experiments, supported the notion that EMR has both particle- and wave-like characteristics, thereby promoting the notion of wave-particle duality.
Lesson Glossary
photoelectric effect: the emission of electrons when a metal is illuminated by EMR with a frequency greater than or equal to the metal’s threshold frequency
photoelectron: an electron emitted from a metal because of the photoelectric effect
stopping voltage: the potential difference for which the kinetic energy of a photoelectron equals the work needed to move through a potential difference
threshold frequency: the minimum frequency that a photon can have to cause photoemission from a metal
wave-particle duality: the notion that EMR has both wave-like and particle-like characteristics
work function: the minimum energy that a photon can have to cause photoemission from a metal
Each metal has a specific work function.