Module 7
1. Module 7
1.14. Page 3
Module 7—Investigating the Nature of the Atom
 Try This
Try This
It is interesting to re-evaluate Rutherford’s planetary model of the atom in light of atomic spectra. Remember that Rutherford’s model violated Maxwell’s laws of electromagnetism. Ignore that flaw for the moment and evaluate Rutherford’s model by investigating what it predicts about atomic spectra.
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Remember to submit your answers to A 1 to your teacher as part of your Module 7: Lesson 3 Assignment.
A 1. According to Maxwell’s laws of electromagnetism, the orbital frequency (the number of complete orbits per second) of an electron will match the frequency of the emitted radiation.
- If an electron were to spiral into the nucleus, what would happen to the electron’s orbital frequency?
- If an electron were to spiral into the nucleus, what would happen to the frequency of the emitted radiation?
- If an electron were to spiral into the nucleus, what kind of spectrum would be produced—a continuous or line spectrum? Explain.
- Look at the emission spectrum of hydrogen. What kind of spectrum is this? Does Rutherford’s model predict the correct spectrum?
 Read
Read
Read “Spectroscopy”on pages 771 to 773 of your physics textbook.
spectroscopy: the study of the light emitted and absorbed by different materials
The Bohr Model of the Hydrogen Atom
At the beginning of the 20th century a model was proposed that finally began to answer some of the questions of atomic structure and spectra. In 1913 Niels Bohr proposed a model of atomic structure using hydrogen as the example model. Bohr’s model not only described the structure of the atom, but it also explained atomic spectra and, furthermore, correctly predicted the existence of more atomic lines. Bohr’s model seemed to be everything that physicists were looking for. But there was one problem—Bohr’s model stepped outside the realm of classical physics and ventured into the newly emerging world of quantum physics. This left many scientists skeptical of the model. Nonetheless, Bohr’s model was far superior to any previous model and was accepted as a semi-classical model of the atom.
Bohr’s Postulates
Bohr started with a planetary model of the atom. However, to sidestep the problems that confounded Rutherford, Bohr made several assumptions, including the following:
- Electrons orbit the nucleus. They are held in orbit by an electrostatic force.
-
Electrons can only be in certain, permitted orbits and an electron does not emit radiation when it is in one of these orbits. In these allowed orbits, the energy of the electron is
constant. These orbits are called stationary states, since the electron’s energy is constant. In other words, the energy of the electron is quantized—it can only have certain values (recall the concept of the “particle-in-a-box” from Module 6: Lesson 3 or page 734 in your textbook). Therefore, the allowed orbits can be referred to as energy states.
stationary state: a stable state with a fixed energy level
energy level: a discrete and quantized amount of energy
- An electron only emits radiation when it “falls” from a higher energy state to a lower state. The change in energy of the electron (from the higher state to the lower state) is equal to the energy of the emitted photon, thereby obeying the conservation of energy principle. Similarly, an electron only absorbs radiation when it "jumps" to a higher energy level. Again, the change in energy of the electron is now equal to the energy of the absorbed photon.
- The radii of the allowed orbits are also quantized since each energy state has a specific radius.
Bohr’s model was allowed to have stationary states because of de Broglie’s work on the wavelength of matter. The electron must have a certain speed in order for the Finward and Felectric to be equal. In order for this to occur, the electron has a specific wavelength, which happens to be equal to the circumference of the stationary state. The circumference of the second stationary state is equal to twice the electron’s wavelength and so on. This agrees with quantum theory. See “Figure 15.24” on page 782 of the textbook.
By applying his assumptions, Bohr was able to develop expressions for the allowed energy levels and the allowed radii for the hydrogen atom. Using these expressions, Bohr calculated all the allowed electron energy levels for hydrogen.
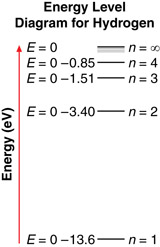
An energy level diagram, like the one shown here, often illustrates energy levels. An energy level diagram displays several things, such as:
- The energy levels of hydrogen are not evenly spaced. As an electron moves to higher and higher levels, the difference in energy between the levels becomes smaller and smaller.
- The energy of each level is reported as a negative number. As an electron moves to a higher energy level, its energy increases (becomes less negative).
- When an electron makes a transition, it moves from one energy level to another. The spacing between energy levels represents the magnitude of the change in energy of the electron. For example, an electron moving from n = 3 to n = 1 has a greater change in energy than an electron moving from n = 3 to n = 2
 Watch and Listen
Watch and Listen
View “Energy Levels of Hydrogen.”
Bohr’s model of the hydrogen atom successfully explained emission and absorption spectra. Not only did Bohr’s model provide a conceptual description of emission and absorption spectra, it also correctly predicted the wavelength of the spectral lines in hydrogen’s emission and absorption spectra. Furthermore, Bohr’s model explained why absorption lines match emission lines.
According to Bohr’s model, an electron only emits or absorbs energy when it moves between energy levels. The energy that is emitted or absorbed by an atom is in the form of a photon.
The amount of energy that is emitted or absorbed is the energy difference between the energy levels. |
The frequency or wavelength of a photon is related to its energy. |
||||||||||||||||||||||||||||||
Expressed as an equation:
|
Expressed as an equation:
|
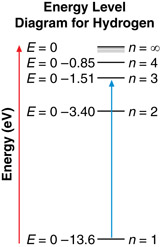
Example Problem 1. A photon is absorbed by a hydrogen atom, causing an electron to jump from the n = 1 energy level to the n = 3 energy level. Using Hydrogen’s energy level diagram, determine the change in energy of the electron and the wavelength of the absorbed photon.
Given
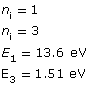
Required
the energy of the released photon and the wavelength of the photon
Analysis and Solution
The red arrow shows that an electron has made a transition from the n = 1 to the n = 3 energy level. The change in the electron’s energy that occurs as a result of the transition is as follows:
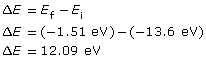
The change in the electron’s energy is equal to the photon’s energy, which is related to its wavelength as follows:
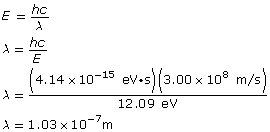
Paraphrase
The energy of the photon is 12.09 eV and it has a wavelength of 1.03×10–7 m.
Watch and Listen
To simulate the example problem transition and see the dark line spectrum that results, open the “Hydrogen Atom Simulation.” On the simulation, press “play” and select the ( ) probability cloud representation. Next, select the n = 3 transition.
) probability cloud representation. Next, select the n = 3 transition.
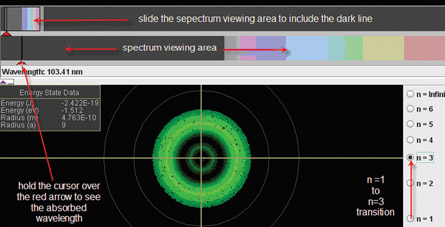
Using this simulation, see if you can verify each of the points below, summarizing the Bohr Model of the atom.
Summary of the Bohr Model
- Absorption (nf > ni). When an electron “jumps” to a higher energy level, it must absorb energy. Each transition requires a specific amount of energy. The dark lines in an absorption spectrum correspond to specific photon wavelengths that are needed for an electron to jump from lower to higher energy levels.
- Emission (nf < ni). When an electron “falls” to a lower energy level, energy is emitted. Each transition emits a specific amount of energy. The lines that are seen in an emission spectrum correspond to specific photon wavelengths that are emitted when an electron jumps from higher to lower energy levels.
- Absorption and emission lines match. For example, the magnitude of the change in energy (ΔE) when an electron rises from n = 1 to n = 2 is equal to the magnitude of the change in energy (ΔE) when an electron falls from n = 2 to n = 1.
-
The absolute value, or the magnitude of the change in energy, is calculated based on the initial and final energy of the electron that undergoes a transition:
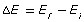
-
The frequency or wavelength of an absorbed or emitted photon can be calculated with
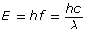 .
.
absorption spectrum: a pattern of dark lines produced when light passes through a gas at low pressure.
emission spectrum: a pattern of bright lines produced by a hot gas at low pressure
 Read
Read
Read “The Bohr Model of the Atom” on pages 773 to 780 of your physics textbook.
 Self-Check
Self-Check
Test your understanding of Bohr’s model of the hydrogen atom by answering the following questions. For questions involving calculations, you may use the Hydrogen Atom Simulation to verify your answers.
SC 3. Look at the assumptions Bohr made. Which of these assumptions "fit" with classical physics and which support the ideas of quantum physics?
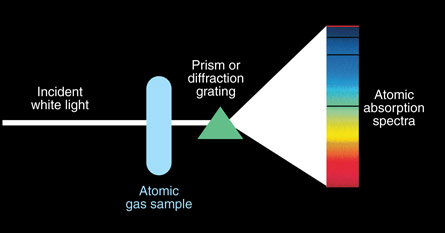
SC 4. Sample equipment to show atomic absorption lines is shown in the diagram below. Absorption lines occur in atomic spectra when an electron absorbs the energy. The electron quickly drops back to the ground state, releasing a photon with the same amount of energy that was absorbed. If the photon is released, why does it not show up on the atomic spectra?
 Self-Check Answers
Self-Check Answers
Contact your teacher if your answers vary significantly from the answers provided here.
SC 3.
| Postulates That “Fit” Classical Physics | Postulates That “Fit” Quantum Physics |
|
|
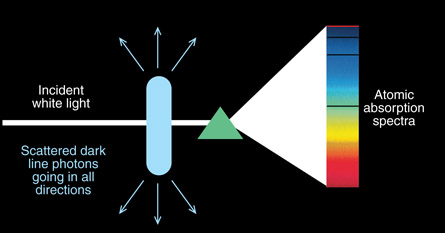
SC 4. The initial incident photons are all projected in one direction through the atomic gas and onto the detector or screen. The photon absorbed by the electron and later released is scattered in a random direction, as shown in the diagram below. As a result, the dark lines are a very few photons re-emitted in that direction but the majority of the photons are emitted in different directions.
Continue to test your understanding of Bohr’s model of the hydrogen atom by answering the following questions. As in the Self-Check activity above, for questions involving calculations, you may use the Hydrogen Atom Simulation to verify your answers.
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Remember to submit your answers to A 2, A 3, A 4, A 5, A 6, A 7, and A 8 to your teacher as part of your Module 7: Lesson 3 Assignment.
A 2. According to Bohr, why did an atom not collapse in on itself while its electrons travelled around the nucleus?
A 3. Using Bohr’s model, explain what happens in the atom when a photon of light is
- emitted
- absorbed
A 4. Earlier, when you read about the energy levels of hydrogen, you were introduced to an energy level diagram. On this diagram, the n = ∞ energy level is represented. On the applet, complete an n = 1 to n = ∞ transition and observe the energy state data.
- According to the applet, what is the energy of the n = ∞ energy level?
- If an electron is initially in the ground state, how much energy must the atom absorb for this transition to occur?
- What is the radius of the n = ∞ energy level?
- What happens to the atom if the electron “jumps” to the n = ∞ energy level? Hint: Look at the radius of this energy level. Is the electron really part of the atom anymore?
A 5. In the hydrogen atom, the electron jumps from the n = 1 level to the n = 4 level.
- During this transition, is a photon emitted or absorbed?
- What is the change in energy of the electron and what is the wavelength of the emitted or absorbed photon?
- Identify the transition by drawing an arrow on the energy level diagram below, and calculate the wavelength of the absorbed photon.

A 6. An electron in the third stationary state around a hydrogen atom has energy of –1.512 eV. What will the electron’s energy be if the hydrogen atom absorbs a photon with a wavelength of 109 nm?
A 7. What is the shortest wavelength photon that is emitted in the hydrogen atom? What transition emits this photon? Hint: If the wavelength is small, then the energy is large. Looking at the energy level diagram will also help you.
A 8. Bohr’s model of the atom explains why emission and absorption lines match up. Prove this for the hydrogen atom. Choose any transition (and its opposite) and calculate the wavelength of the emitted and absorbed photon. Verify your answer using the applet.
 Try This
Try This
TR 4. Use this Bohr Model of Hydrogen Simulation to shoot a stream of photons through a container of hydrogen gas. Observe how photons of certain energies are absorbed, causing changes in the orbits of electrons. Build the spectrum of hydrogen based on photons that are absorbed and emitted.
TR 5. You can also use this Bohr Model: Introduction Simulation to fire photons and observe how an absorbed photon changes the orbit of an electron, and how a photon is emitted from an excited electron. Calculate the energies of absorbed and emitted photons based on energy level diagrams. The light energy produced by the laser can be modulated, and a lamp can be used to view the entire absorption spectrum at once.