Module 7
1. Module 7
1.12. Lesson 3
Module 7—Investigating the Nature of the Atom
Lesson 3—The Rutherford and Bohr Models of the Atom
 Get Focused
Get Focused
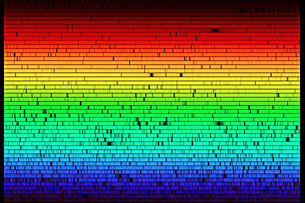
Image courtesy of the National Optical Astronomy Observatory/Association of Universities for Research in Astronomy/National Science Foundation
This image shows the full visible spectrum of colours emitted by the surface of the Sun as observed at the McMath-Pierce Solar Observatory. Recall from Module 5 that when full-spectrum white light passes through a prism, it is dispersed into a spectrum as each wavelength refracts at a slightly different angle. Notice that there are some missing wavelengths, seen as black spots, and that the sunlight is composed mostly of yellow-green wavelengths.
At first glance, the dark spots in this spectrum seem random and unrelated to one another; but they are, in fact, strong evidence that the Sun’s surface is composed of approximately 74% hydrogen and 25% helium. The relative amount and presence of these elements is related to the nuclear reactions that generate the Sun’s energy. In a similar way to that of a mass spectrometer analyzing the composition of Titan’s atmosphere, knowledge of the far and wide originated from an understanding of the close-up and invisibly small. As ideas of the atom evolved from Thomson’s raisin-bun to Rutherford’s planetary system and eventually to Bohr’s concept of the stationary state, the colour of light emitted or absorbed by matter has taken on more significance, hinting at the very composition of the matter with which it interacts!
In Lesson 3 you will explore the fundamental makeup of matter that is revealed by its interaction with electromagnetic radiation and charged particles.
In this lesson you will answer the following essential questions:
- What does the Rutherford Scattering Experiment suggest about the nature of the nucleus, and how did it lead to the planetary model of the atom?
- What is the Bohr model of the atom, and how is the concept of stationary states and energy quantization used to explain how a gas absorbs and emits only certain wavelengths of electromagnetic radiation?
 Module 7: Lesson 3 Assignment
Module 7: Lesson 3 Assignment
Your teacher-marked Module 7: Lesson 3 Assignment requires you to submit responses to the following:
- Lab—LAB 1 and LAB 2
- Assignment—A 1, A 2, A 3, A 4, A 5, A 6, A 7, and A8
The other questions in this lesson are not marked by the teacher; however, you should still answer these questions. The Self-Check and Try This questions are placed in this lesson to help you review important information and build key concepts that may be applied in future lessons.
After a discussion with your teacher, you must decide what to do with the questions that are not part of your assignment. For example, you may decide to submit to your teacher the responses to Try This questions that are not marked. You should record the answers to all questions in this lesson and place those answers in your course folder.